Our Science
Biology of Aging


Over the past decade a growing body of scientific evidence has demonstrated the critical role of senescent cells in driving cellular aging and age-related diseases. By selectively clearing the body and tissues of these cells, while preserving healthy cells, the tissues can rejuvenate and disease progression can be reversed to restore cellular health.
At Rubedo, our Alembic drug discovery platform enables us to identify specific druggable targets and then develop them into therapeutics that selectively target the pathologic cells (such as senescent cells) that drive the process of aging. This allows us to address the fundamental cause of age-related diseases and preserve biological youth.

Alembic Platform
Our proprietary ALEMBIC™ AI-driven drug discovery platform is based on a synergy between sophisticated computational algorithms and chemistry. Rubedo’s differentiated technology is a therapeutic agnostic platform aimed at addressing a wide variety of diseases beyond senescence and aging.
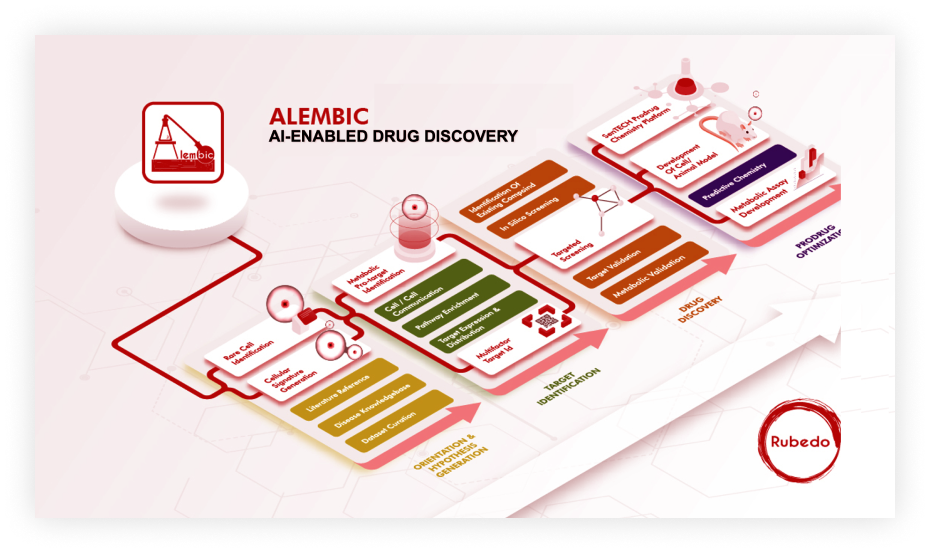
How we are differentiated

HOW IT WORKS